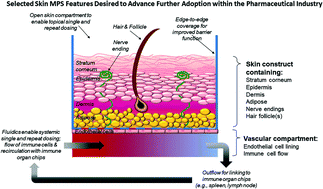
Skin toxicology testing seeks to assess toxins within substances that could affect the integrity and function of human skin. Toxins coming into contact with the skin can result in redness, heat, pain and swelling of the area; identifying where this may happen is covered by a range of skin toxicology tests.
Various OECD assay tests ascertain whether a test substance is likely to cause corrosion, irritation, sensitisation, and phototoxicity to human skin. Testing is carried out using cultured tissues or mammalian cells, and to a reducing extent, in animals or human patch tests.
Each test is carried out under OECD Test Guidelines for Chemicals, an internationally agreed set of the most relevant testing methods used by independent laboratories, industry and governments to characterise the potential hazards of chemicals. Now widely used in toxicology research, there are several appropriate tests you may need to carry out.
Gentronix is a predictive toxicology company using the latest approved in vitro testing assays to assist companies requiring comprehensive assessments of dermal toxicity within substances. It is essential to identify the potential for harm to the skin caused by useful substances such as therapeutics, cosmetics or industrial chemicals. Carrying out relevant testing ensures product safety.
Here is a rundown of the main assay tests available for in vitro skin toxicology testing.
OECD 431 3D Skin Corrosion
This test uses in vitro procedures requiring reconstituted human skin 3D modelling to assess the test substance corrosivity to human skin under OECD 431. Tissue must comprise a reconstructed epidermis with a functional stratum corneum, with two tissues samples used to identify corrosives materials that decrease cell viability below predefined thresholds over specified exposure periods.
OECD 439 3D Skin Irritation
OECD 439 is very similar to OECD 431, as it again uses human skin 3D modelling. The OECD 439 test can assess the potential of the test substance to irritate human skin. This test may stand alone as a replacement test for in vivo skin irritation testing or within a tiered testing strategy.
OECD 432 Phototoxicity
The OECD 432 test assesses cytotoxicity induced after a test substance is exposed to UV light. In vitro 3T3 neutral red uptake phototoxicity testing enables identifying elements likely to cause chemically induced skin damage requiring light that does not involve the immune system.
OECD 442D KeratinoSens
The OECD 442D assay assesses the second key event of skin sensitisation adverse outcome pathways – the activation of keratinocytes. The expression of a luciferase gene reporter is measured for ARE-dependent pathways, as the first step in skin allergenicity is the covalent binding of a substance to skin proteins.
OECD 442C DPRA
Utilising HPLC, this assay detects the potential for reactivity of a test substance to lysine peptides and cysteine. Depletion of these peptides identified by test substance reactivity is used to support the discrimination between skin sensitisers and non-sensitisers.
OECD 442E h-CLAT
The third key event of the skin sensitisation adverse outcome pathways – dendritic cell activation is investigated by the OECD 442E h-CLAT assay. Flow cytometry is used to assess the ability of a test substance to increase surface markers, leading to the stimulation of an immune response required for sensitisation of the skin to the test substance.
All of these important tests are vital in determining what effects materials have on skin, allowing manufacturers to ensure that their products do not cause damage to those that use them.
Help keep news FREE for our readers
Supporting your local community newspaper/online news outlet is crucial now more than ever. If you believe in independent journalism, then consider making a valuable contribution by making a one-time or monthly donation. We operate in rural areas where providing unbiased news can be challenging. Read More About Supporting The West Wales Chronicle